A green energy revolution is well underway, but the speed bumps are formidable. Consider the challenge of energy storage. Wind turbines only generate electricity when the wind blows, and solar panels when the sun shines. This intermittency makes large-scale energy storage a necessity, but current technologies have significant limitations.
Today, the batteries used for this type of storage rely on metals such as lithium and cobalt, which are energy intensive to mine and have a relatively limited lifespan. The same materials are used in electric vehicle batteries and have not been able to match the range of their petroleum-powered counterparts.
Advanced nanostructured materials could flip the script. Through a process called solid-state synthesis, nanotechnologists are creating purpose-built crystals, manipulating their structure to achieve specific properties. One of the leading lights in this emerging field is Elena Meirzadeh, an assistant professor at the Weizmann Institute of Science and Azrieli Early Career Faculty Fellow.
Meirzadeh was born in Tehran and moved to Israel when she was 11 years old. But she did not have a strong command of either Hebrew or English, and it took time for her to adjust to life in Israel. She didn’t even take to science right away, and almost gave up on chemistry in high school. But in her final year, she was inspired by the hands-on scientific approach of her chemistry teacher, who taught science as a process of experimentation rather than a theoretical endeavour. That captured Meirzadeh’s imagination and remains central to her research approach.
Working in her lab, Meirzadeh synthesizes crystals composed of atomically precise molecular structures known as superatoms that could be deployed in a variety of applications. One day, these carbon-based molecules could vastly improve energy transport and storage. The optoelectronic properties of superatoms could also make them useful for quantum computing transistors. Some are even superconductors, and this could have all kinds of potential applications if technical challenges can be overcome.
“I’m fascinated by the mechanisms of the formation of crystals — how to make them and incorporate interesting properties into their design,” says Meirzadeh.
It’s easy to get caught up in her enthusiasm. By controlling the parameters of solid-state synthesis and exposing materials to various external stimuli, Meirzadeh can “tune” her crystals to achieve specific magnetic or electrical properties. She does this using a synthetic approach she developed called pyrolytic vapour polymerization reaction. The technique seals the molecular precursors for a new crystal inside a vacuum tube made of quartz, which is then baked in a specialized oven. The precursors vaporize and react, and as they cool, they solidify into a new crystal.
It’s a powerful technique that allows scientists to grow large, high-quality single crystals, Meirzadeh says. Using a combination of experience and intuition, she can manipulate various parameters to achieve specific goals. “If we can grow single crystals, determine their structure and measure their properties, then we can know that a specific structure results in a specific property,” says Meirzadeh. “And we can change a structure to get that property and basically design whatever we want. But that’s really hard to do.”
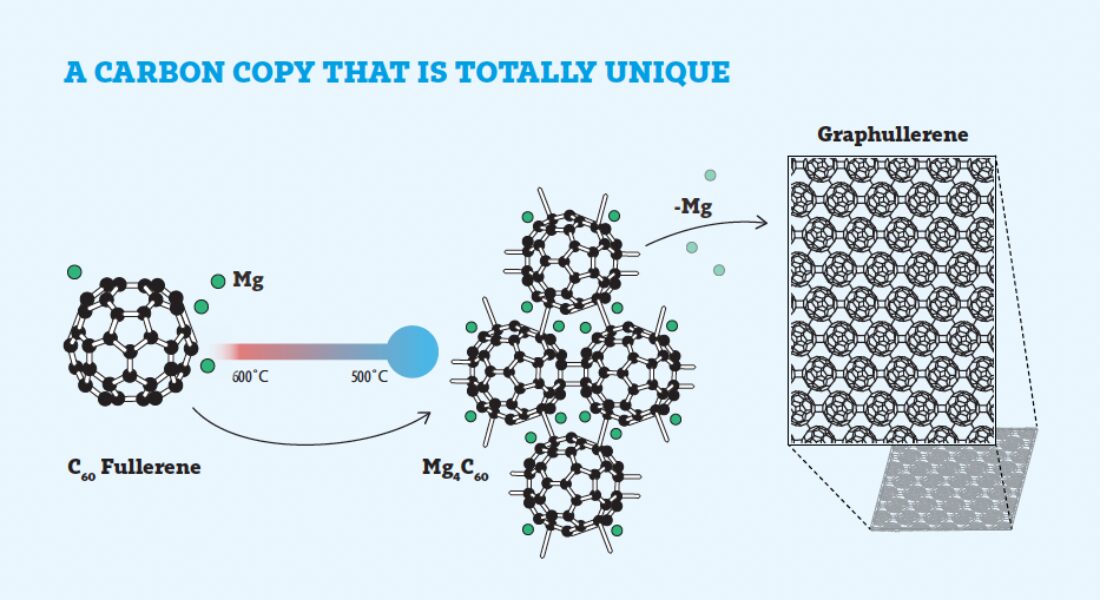
The oven Meirzadeh uses to cook new materials has zones for heating and cooling that can be precisely controlled. And even after the molecular precursors crystallize into a unified material, its exact identity is still a mystery. It could be something already known to science, and most of the time it is. But the process can also create something entirely novel, and Meirzadeh needs to characterize the new crystal to determine whether it is. During her postdoctoral research at Columbia University, Meirzadeh created a superatomic two-dimensional material called graphullerene — by happenstance — that exhibits promising thermal conductivity properties (see related story).