It’s easy to take water for granted. Throughout much of the world, a twist of the tap yields the instant reward of clean, abundant water, usually at minimal cost. But the process of getting that water from source to glass is staggeringly complex, involving hundreds of engineering solutions along the way.
In some places, concerns about cleanliness must be addressed because water travels through agricultural fields, small settlements or ancient pipes, picking up contaminants on its journey. In addition to disease-generating bacteria, water is often polluted with pesticides, pharmaceuticals and dyes from textile processing. The United Nations estimates that two billion people around the globe do not have access to safe drinking water and that at least half of the planet’s population experiences water scarcity at least once a year. Around 17 million women give birth annually in health care facilities with inadequate water, and 206 million people spend more than half an hour per trip collecting water.
“Where I grew up, there is no 24-hour-per-day water supply,” Kirti Sankhala says about her hometown, Jodhpur, in India’s arid northern state of Rajasthan, where she works as an assistant professor in the Center for Emerging Technologies for Sustainable Development at the Indian Institute of Technology Jodhpur (IIT Jodhpur). “Sometimes we only get water once every two days. But water culture is different here. Everyone has a storage tank, so when water is supplied, people store it.
“We all want a safe glass of water,” continues Sankhala, who was an Azrieli International Postdoctoral Fellow in the Department of Chemical Engineering at Technion–Israel Institute of Technology in 2021 and 2022. “We want high purity — the rejection of contaminants that are a health threat — and at the same time we also want high productivity, which means generating more clean water using less energy and time.”
At IIT Jodhpur, her new research group is contributing toward objective number six on the United Nations’ list of sustainable development goals: ensure availability and sustainable management of water and sanitation for all. For Sankhala, that means providing sustainable water treatment solutions using local natural resources, such as sand, clay and plants, as well as applying emerging membrane technology for water treatment.
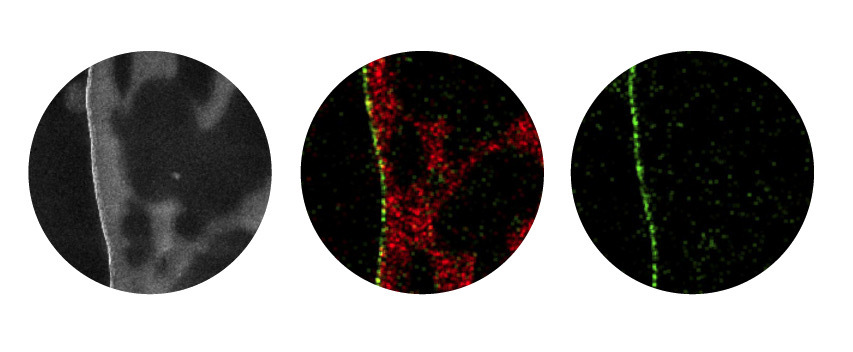
Her research focuses on nanofiltration, in which water is forced through microscopic pores to filter out contaminants at a molecular level. The pores in these membranes are between one and 10 nanometres in size. (For comparison, a human hair is 100,000 nanometres across, and a bacterium is 2,000 times larger than the smallest pores in Sankhala’s membranes.) Cleaning water with a membrane, then, is like making coffee in the morning, she says: “You pour everything into the filter on top and then only your coffee will come out, but all the particles will stay on top of the sieve.”