Twenty years ago, a pair of researchers at the University of Manchester made a remarkable breakthrough in their lab using an everyday adhesive. While attempting to isolate a layer of carbon that was as thin as possible, they looked through a microscope at the grey, slightly shiny residue from a graphite crystal left behind on a piece of Scotch tape. What they saw was graphene, the first two-dimensional material ever discovered, a eureka moment that eventually led to the Nobel Prize in Physics.
Stronger than steel but lighter than paper, graphene conducts electricity more efficiently than copper wire. Virtually transparent, it also conducts heat better — and is thinner — than any other material. These attributes give graphene almost limitless potential, once we figure out how to use it more effectively.
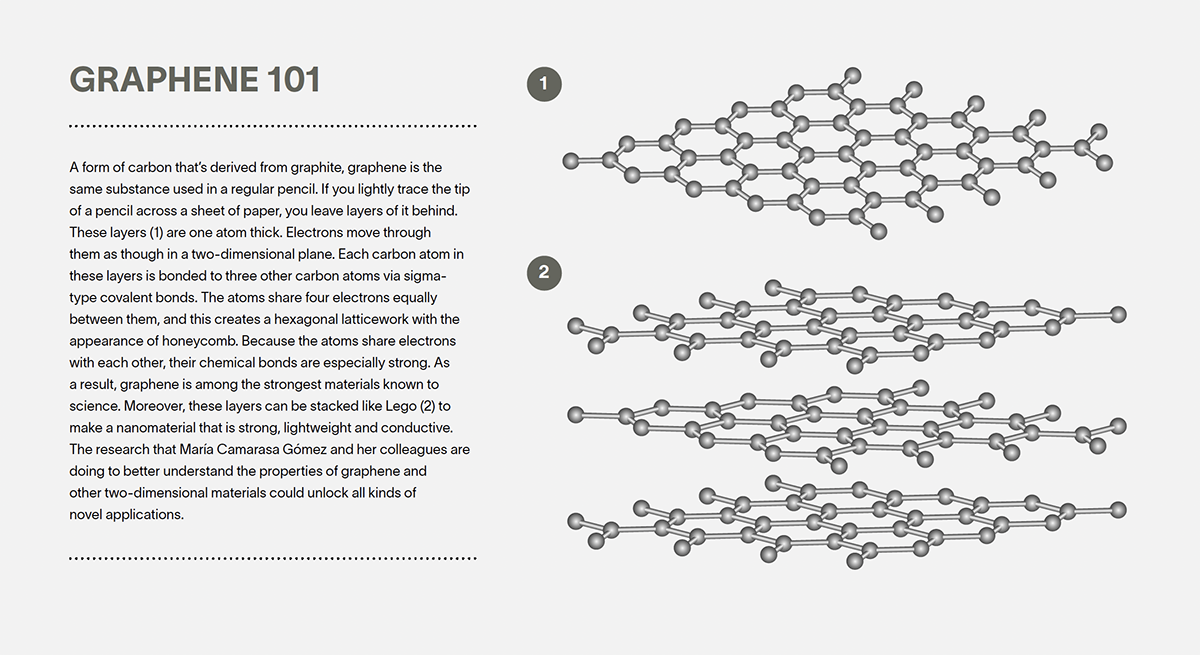
A form of carbon that’s derived from graphite, graphene is the same substance used in a regular pencil. If you lightly trace the tip of a pencil across a sheet of paper, you leave layers of it behind. These one-atom-thick layers can be stacked like Lego to make a nanomaterial that is strong, lightweight and conductive — and that could unlock all kinds of novel applications.
Already a strong candidate for several innovative technologies, graphene could one day be used to make a mobile phone so bendable that you could wrap it around your wrist like a watch, then seamlessly reform it into a rectangle. It could improve the efficiency of solar panels and batteries, enabling electric vehicles to travel tremendous distances on a single charge. It even has the potential to be used in biosensors that could detect cancerous tumours earlier and deliver targeted therapies directly to them before it’s too late. But we can’t do these things yet. To make the most of graphene, we need a fuller understanding of how it works on a molecular level.
Graphene is just one example of a two-dimensional material. These materials are made of ultra-thin layers just a single atom thick, and they can have all kinds of exciting properties. They have the potential to transform numerous industries, but it is difficult to understand exactly how they work, and even more difficult to harness their potential. Researchers like María Camarasa Gómez are confronting this challenge.
The Spanish physicist, who operates at the confluence of quantum mechanics and theoretical chemistry, joined the quantum theory of materials research group at the Weizmann Institute of Science in August 2021 as an Azrieli International Postdoctoral Fellow. Led by principal investigator Leeor Kronik, the group aims to predict the properties of materials based only on their atomic composition and the laws of quantum mechanics.