Imagine a world where crops can clean extra carbon dioxide out of the air and help reduce global warming. Where modified solar panels produce energy that can either be used now or stored for later. And where industry uses novel catalysts that make chemical processes far cleaner than ever before. These seemingly unrelated ideas have one thing in common: they could all contribute to creating a more environmentally sustainable world. They are also getting closer to becoming reality, thanks to research projects supported by the Azrieli Fellows Program.
Adding a second contact to the back (bottom) of the HPEV solar cell enables the collection of electrical energy in addition to the clean chemical energy typically produced by solar cells.
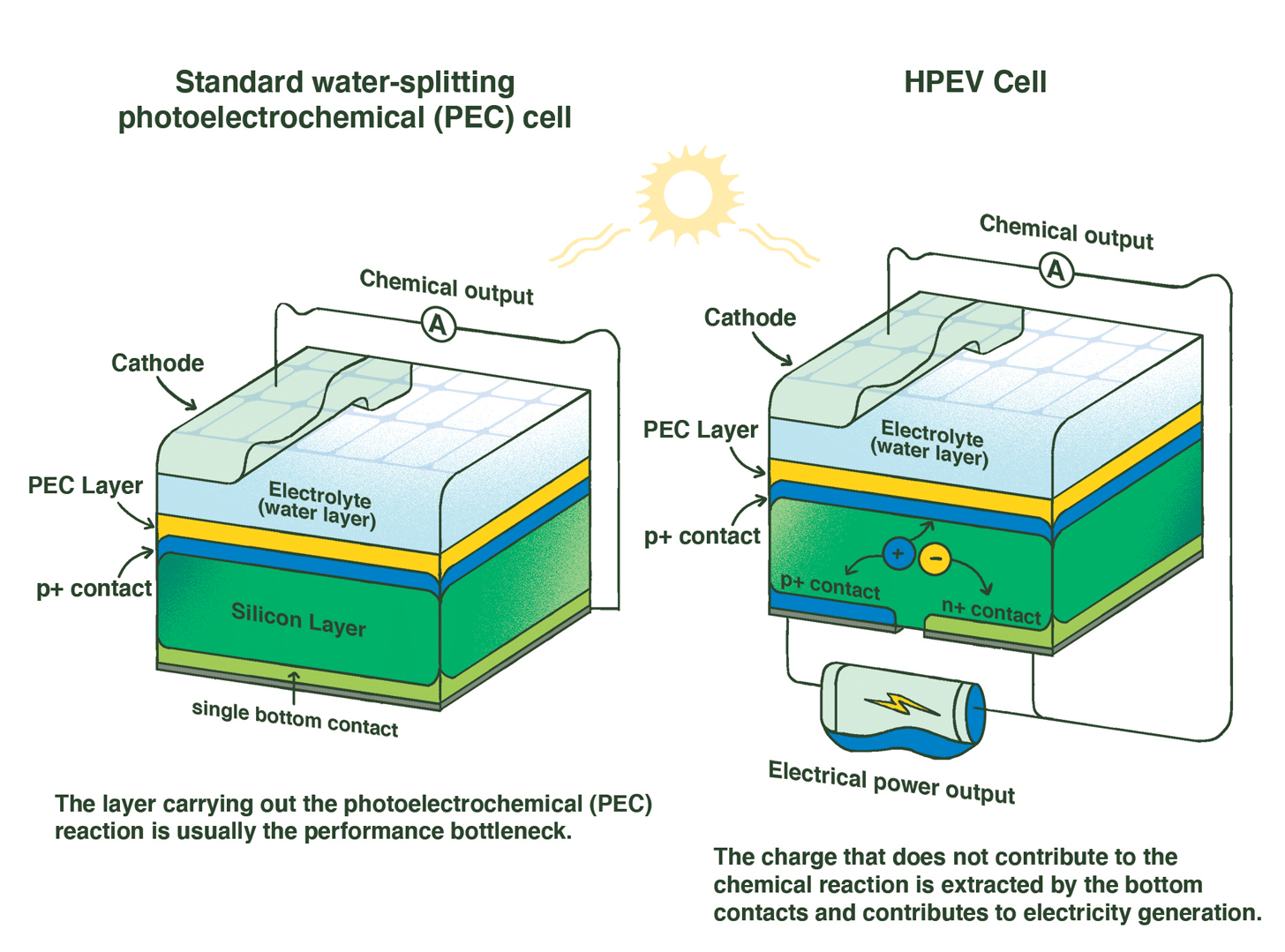
Tel Aviv University researcher Gideon Segev has developed a hybrid solar cell that creates electricity from sunlight while also producing hydrogen gas that can be stored and used later to generate clean energy. Photograph by Shauli Lendner

Devin Trudeau co-developed a new-to-nature plant metabolic pathway that could improve crop yields, leading to more efficient use of farmland for both food and biofuel products. Photograph by Shauli Lendner

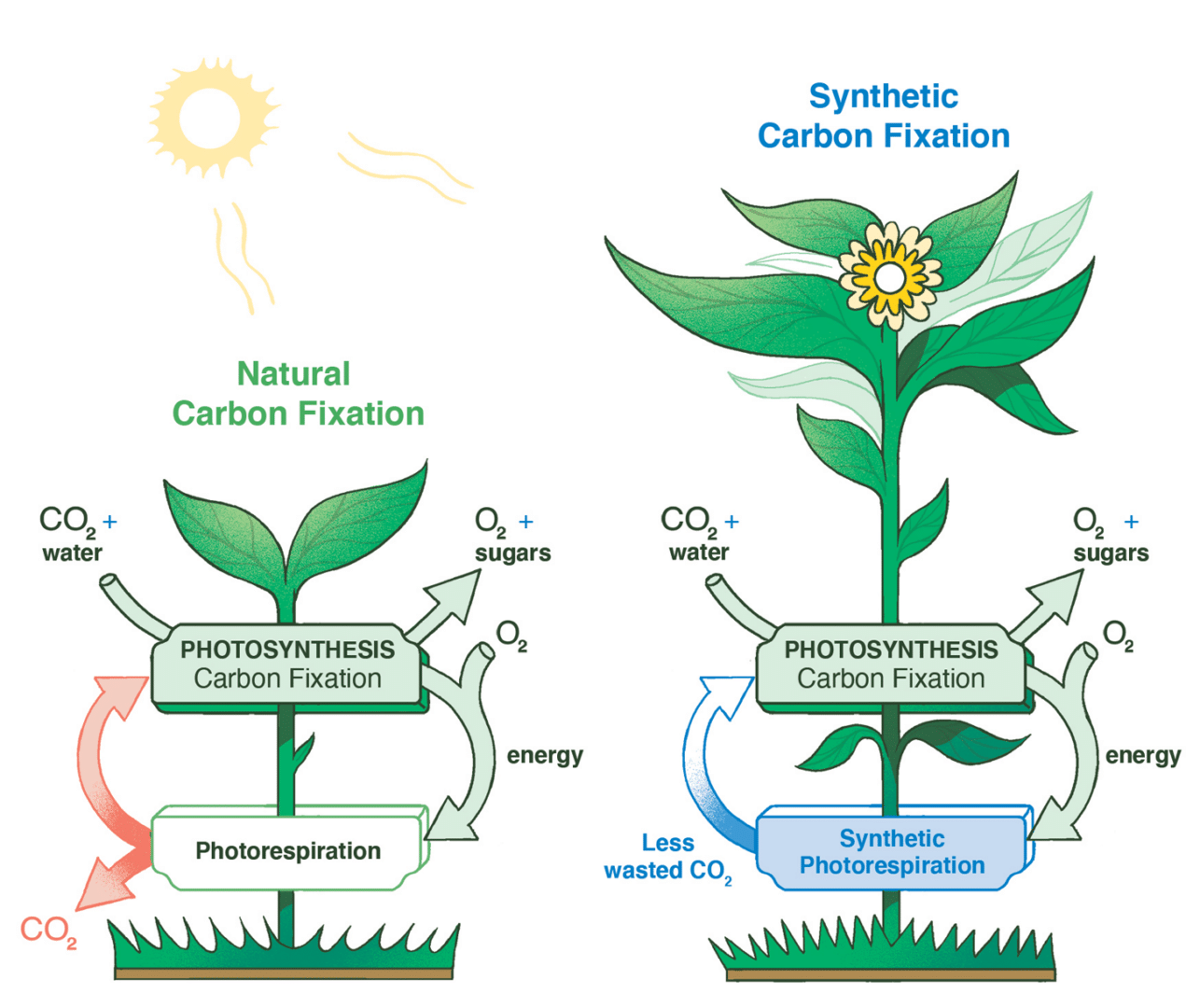
A new bioengineered pathway allows plants to conserve CO2, and thus grow more efficiently and productively than their natural counterparts under the same circumstances.
At the Laboratory for Inorganic & Materials Chemistry at the Technion – Israel Institute of Technology, Graham de Ruiter is researching sustainable organic transformations involving earth-abundant metals. Photograph by Nitzan Zohar, Technion Spokesperson’s Office
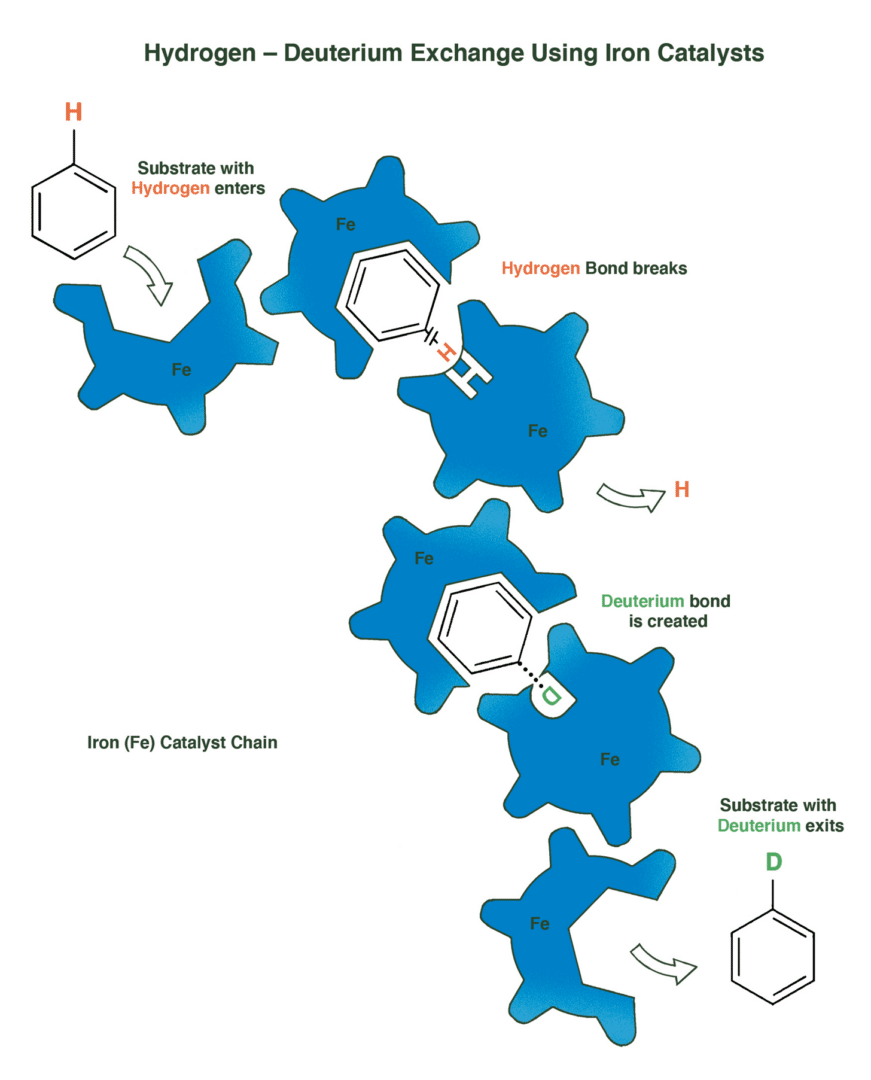
Using iron catalysts in chemical reactions provides a less toxic and more efficient pathway for isomerization (rearranging molecules’ structures to create industrially useful compounds).