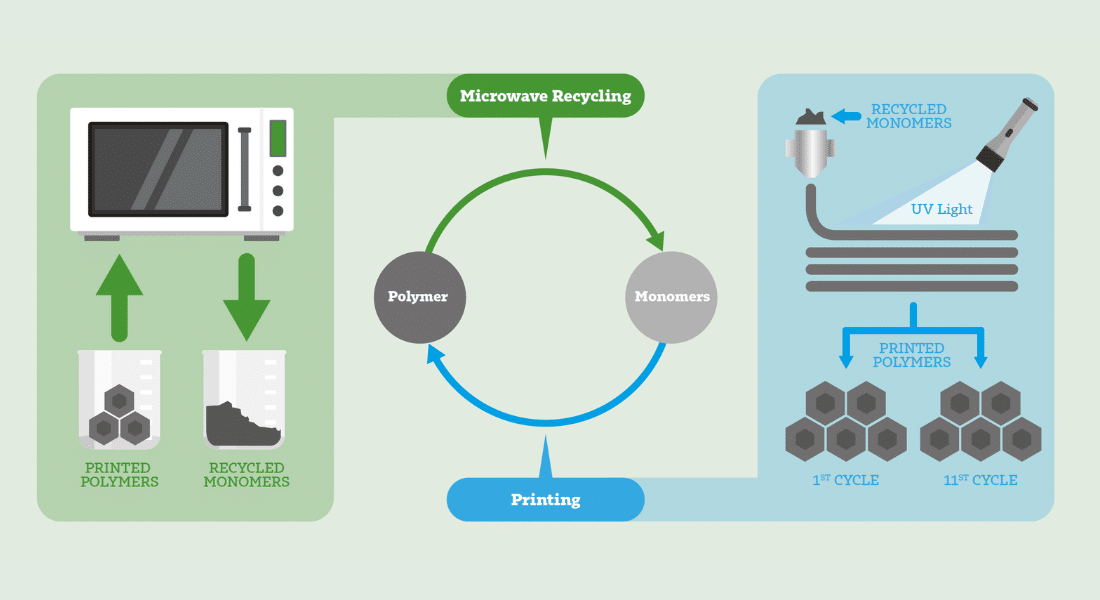
We have a big plastic problem. Actually, there are many problems throughout the plastic life cycle. Plastic production emits vast amounts of greenhouse gases. Most of the plastic in circulation is in single-use products that quickly make their way to landfill and beyond. And every year, up to 23 million tonnes of plastic waste leak into aquatic ecosystems. To top it off, plastic is notoriously difficult to recycle. But Natanel Jarach believes we may be able to do something about that last challenge.
“We live in the era of plastic,” says Jarach, a PhD candidate and Azrieli Graduate Studies Fellow at the Institute of Chemistry at the Hebrew University of Jerusalem. “Look around you — everything is made of plastic, or polymers to be more precise.” Polymers are natural or synthetic substances made of large molecules that are, in turn, composed of smaller, simpler chemical units known as monomers. “We can’t live without them,” he adds, “but they’re causing damage to the environment because most of them are not degradable and can’t even be recycled.”
Born and raised in Israel, Jarach earned both his bachelor’s and master’s degrees from Shenkar College for Engineering, Design and Art in Ramat Gan, just east of Tel Aviv, where he specialized in polymeric material engineering. His current work, in Shlomo Magdassi’s research group at the Hebrew University, is focused on developing plastics that can be used over and over again.
Traditionally, there have been two main approaches to recycling plastic: mechanical and chemical recycling. Mechanical recycling involves grinding and heating plastic to enable reprocessing. While it is a promising method, it can only process thermoplastics — plastics that are made of polymer chains held together by physical interactions, not chemical bonds. Thermoplastics become soft and mouldable when heated and harden upon cooling. The chemical recycling approach similarly only works with thermoplastics, primarily polyethylene terephthalate (PET), the kind of plastic used in beverage bottles.
In contrast to traditional recycling methods, the method Jarach and his colleagues are developing can work with both thermoplastics and thermosets. Thermosets are plastics that strengthen when cured, forming a chemically bonded network-like structure, such as epoxy and silicone. Unlike thermoplastics, once hardened, thermosets cannot be reheated and reshaped.
A key issue, Jarach notes, is that traditional recycling process causes degradation. What starts off as a rugged, versatile material may end up as a product with more limited use, a phenomenon known as downcycling. “Take a polypropylene plastic chair, which a lot of people have in the home,” says Jarach. “When you recycle it, you might end up with a fruit basket, because the degradation of the material is so significant.”